|
 he steam reforming of methane to produce
synthesis gas is a particularly important reaction, due to the increased availability of natural gas as a
feedstock. An increasing emphasis is on the manufacture of liquid fuels from remote or
stranded natural gas using Fischer Tropsch chemistry, and the generation of hydrogen from natural gas liquid fuels to power
mobile and stationary fuel cells. The syngas generation section of such plants comprises over 50% of the capital cost.
There is thus a strong economic incentive to develop more efficient steam reforming technology, and a major step in this
effort is the optimal design of catalyst particles to achieve a balance between the demands of high catalyst activity, low
pressure drop in the tube, and high heat transfer rates. he steam reforming of methane to produce
synthesis gas is a particularly important reaction, due to the increased availability of natural gas as a
feedstock. An increasing emphasis is on the manufacture of liquid fuels from remote or
stranded natural gas using Fischer Tropsch chemistry, and the generation of hydrogen from natural gas liquid fuels to power
mobile and stationary fuel cells. The syngas generation section of such plants comprises over 50% of the capital cost.
There is thus a strong economic incentive to develop more efficient steam reforming technology, and a major step in this
effort is the optimal design of catalyst particles to achieve a balance between the demands of high catalyst activity, low
pressure drop in the tube, and high heat transfer rates.
This has led us to examine the detailed effects of design changes in the catalyst pellets on transport between particle and
surrounding fluid, and on reaction inside the pellets. To date, however, commercial CFD codes have not been developed to include
reaction inside solid catalysts. To get an idea of how catalyst pellets behave in low-N fixed beds when reaction is present,
we had to extend existing methods to be able to simulate intraparticle transport and reaction
[Dixon, A.G., Taskin, M.E., Nijemeisland, M., and Stitt, E.H., 2010].
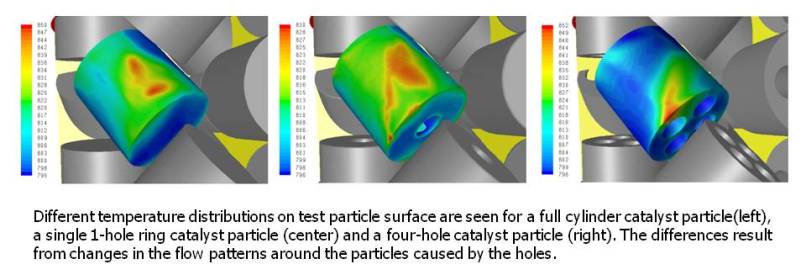
Our objective is the more realistic treatment of catalyst particle internal transport and reaction processes by linking them to the conditions in the
fluid flowing past and surrounding the particle, using CFD. Our work has given results for full cylinders on the effects of external gas flow on the
supply and consumption of reactants in steam reforming and propane dehydrogenation [Taskin, M.E., Troupel,
A., Dixon, A.G., Nijemeisland, M., and Stitt, E.H., 2010].
More recently, we have extended this analysis to shaped cylindrical particles with one, three, four and six internal holes. These holes are used to
provide more surface area for reaction in steam reforming and also they increase void fraction which reduces pressure drop over the reactor
[Dixon, A.G., Boudreau, J., Rocheleau, A., Troupel, A., Taskin, M.E., Nijemeisland, M., and Stitt, E.H., 2012].
We have also extended our CFD methods to be able to include carbon formation reactions, and have illustrated this through calculations on the propane
dehydrogenation process, in which the time evolution of carbon inside the catalysts could be followed, showing the strongly non-symmetric patterns
seen in the near-wall pellets. [Behnam, M., Dixon, A.G., Nijemeisland, M., and Stitt, E.H., 2010]. CFD methods
are also being applied by our group to methane steam reforming catalyst deactivation.
Current research interests include the extension of our CFD methods to include microkinetics and improved multicomponent diffusion models inside the catalysts
and their application to exothermic partial oxidation reactions, such as ethylene epoxidation and maleic anhydride production.
|